Multiplexing Molecular Tests
Inhibitor-tolerant qPCR & LAMP Master Mixes and Enzymes for Respiratory Testing
Direct Detection for Molecular Respiratory Assays
The number of Food and Drug Administration (FDA)–cleared molecular diagnostics for acute respiratory tract infections has increased significantly over the last decade. Studies have shown that multiplex molecular testing for multiple respiratory viruses can be more cost-effective than traditional antigen- or culture-based methods. However, respiratory specimen samples, such as sputum, nasal swabs, and saliva, contain inhibitors that can interfere with PCR amplification by interacting directly with the target DNA and blocking the activity of the polymerase or other PCR mixture components. In order to prevent this interference, RNA or DNA is typically first extracted from the sample before PCR is performed.
As the demand increases for new assays that are more user-friendly, lower in cost, and can deliver faster sample-to-results, molecular methods that use crude samples and do not require DNA or RNA extraction are expected to become common practice. Combining direct detection with non-invasive sample collection using specimens such as saliva, provides the ideal combination for next-generation screening assays. However, assay performance must remain on par with current methods in terms of sensitivity and reliability in order to improve patient care and clinical outcomes.
Point-of-Care MDx Using Saliva-Specific Mixes

Air-Dryable™ Direct RNA/DNA qPCR Saliva: Sensitive qPCR detection in Multiplex Assays Using Samples Containing Universal Transport Media (up to 35% UTM)
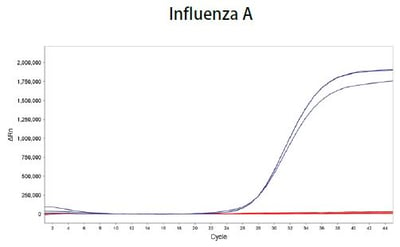
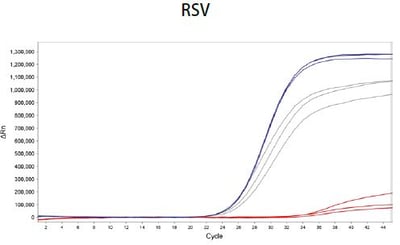
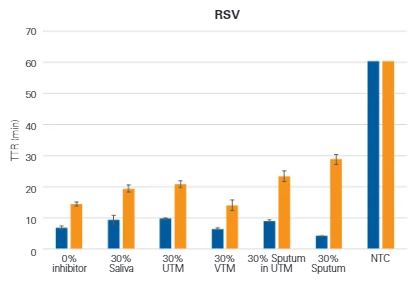
Lyo-Ready™ Direct RNA/DNA LAMP Saliva: High Inhibitor Tolerance and Fast Amplification Delivers Quicker Time-to-Results (TTR) Using Saliva, Sputum and Samples Containing Universal Transport Media (UTM)
Additional Mixes & Enzymes for POC Respiratory Tests